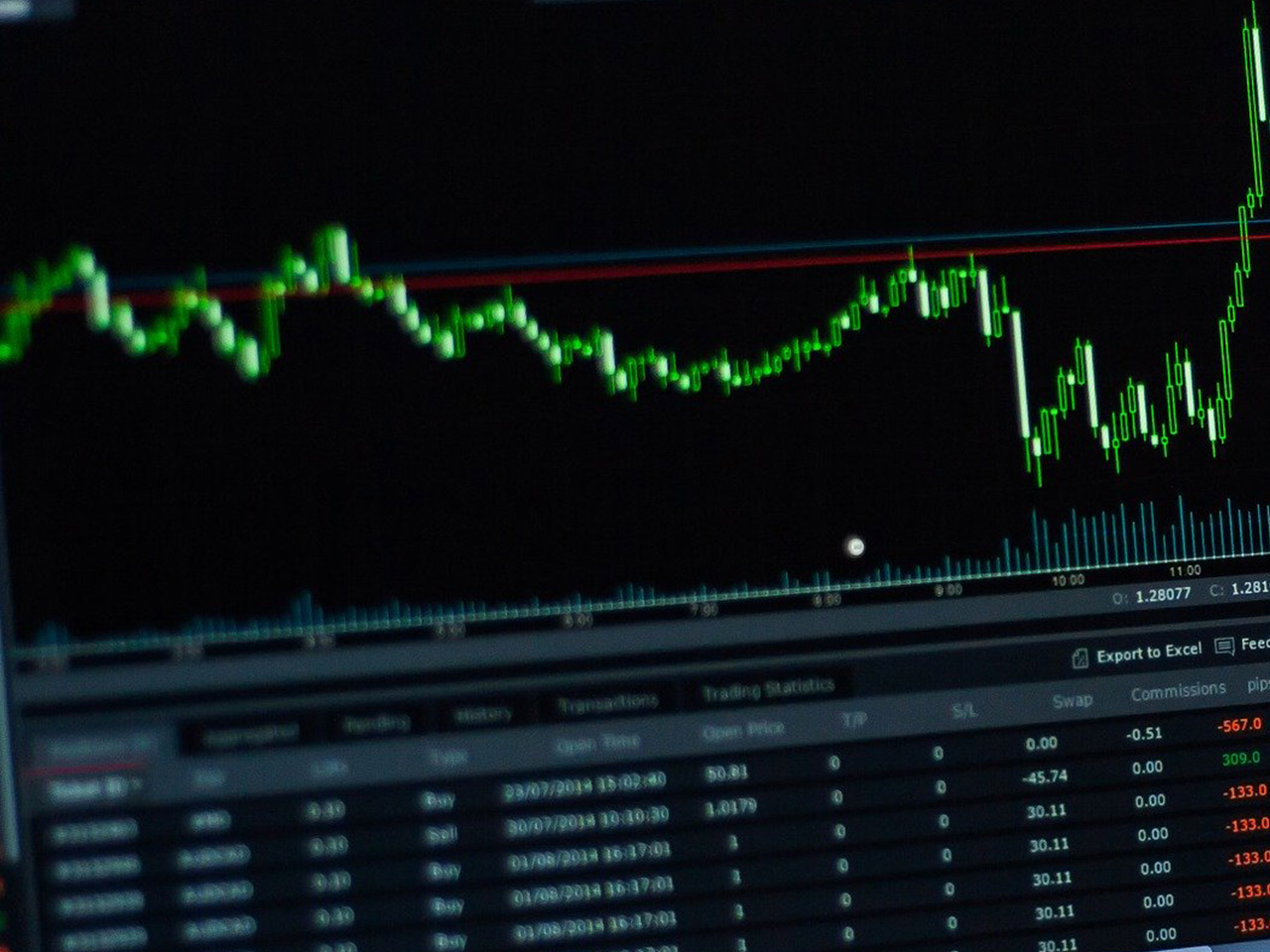
Inovio Pharmaceuticals with ticker code (INO) have now 4 analysts covering the stock with the consensus suggesting a rating of ‘Hold’. The range between the high target price and low target price is between 3 and 2 and has a mean target at 2.25. Now with the previous closing price of 1.52 this now indicates there is a potential upside of 48.0%. There is a 50 day moving average of 1.93 and the 200 day MA is 2.19. The market capitalisation for the company is $407m. Find out more information at: https://www.inovio.com
The potential market cap would be $602m based on the market concensus.
Inovio Pharmaceuticals, a biotechnology company, focuses on the discovery, development, and commercialization of DNA medicines to treat and protect people from diseases associated with human papillomavirus (HPV), cancer, and infectious diseases. Its DNA medicines platform uses precisely designed SynCon that identify and optimize the DNA sequence of the target antigen, as well as CELLECTRA smart devices technology that facilitates delivery of the DNA plasmids. The company engages in conducting and planning clinical studies of its DNA medicines for HPV-associated precancers, including cervical, vulvar, and anal dysplasia; HPV-associated cancers, such as head and neck, cervical, anal, penile, vulvar, and vaginal; other HPV-associated disorders, including recurrent respiratory papillomatosis; glioblastoma multiforme; prostate cancer; HIV; Ebola; Middle East Respiratory Syndrome (MERS); and Lassa fever. Its partners and collaborators include ApolloBio Corp., AstraZeneca, Beijing Advaccine Biotechnology Co., Ltd., The Bill & Melinda Gates Foundation, Coalition for Epidemic Preparedness Innovations (CEPI), Defense Advanced Research Projects Agency (DARPA), Department of Defense (DoD), HIV Vaccines Trial Network, International Vaccine Institute, Kaneka Eurogentec, Medical CBRN Defense Consortium (MCDC), National Cancer Institute, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Ology Bioservices, the Parker Institute for Cancer Immunotherapy, Plumbline Life Sciences, Regeneron Pharmaceuticals, Thermo Fisher Scientific, University of Pennsylvania, Walter Reed Army Institute of Research, and The Wistar Institute. The company has an agreement with Richter-Helm BioLogics GmbH & Co. KG to support investigational DNA vaccine INO-4800 for COVID-19; and a partnership with International Vaccine Institute and Seoul National University Hospital. The company was founded in 1979 and is headquartered in Plymouth Meeting, Pennsylvania.