EDX Medical Group plc (AQSE:EDX), which develops innovative digital diagnostic products and services supporting personalised treatments for cancer, cardiovascular and infectious diseases, announced today it has developed a new ‘super test’ for prostate cancer in an effort to revolutionise screening and diagnosis of the disease and accelerate personalised treatment for patients.
The EDX test identifies the presence or absence of cancerous cells, signs of early and late-stage cancer, whether it is slow or aggressive as well as genetic and hereditary risks in the patient.
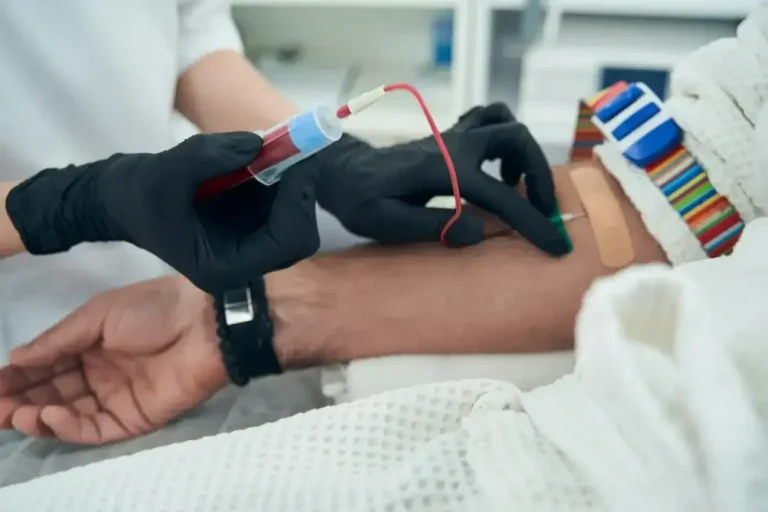
The EDX test involves studying the most comprehensive combination of clinically-validated prostate-related biomarkers currently known, in both blood and urine samples. The interpretation of these biomarkers using a proprietary AI-driven algorithm highlights early signs of cancer and characteristic features that can guide treatment selection.
More than 100 clinically validated biomarkers are measured in the EDX test. The biomarkers are then analysed by the AI-powered algorithm which creates a detailed report of results for doctors. Currently available advanced tests rely on up to 20 biomarkers per test.
The company has filed a patent application for the test and the AI algorithm with the European Patent Office. EDX Medical’s scientific team will validate further clinical data over coming months prior to seeking regulatory approval from the Medicines & Healthcare Products Regulatory Agency (MHRA) and the US Food and Drug Administration (FDA) with a view to launching the test later this year or early 2026.
There are 55,000 new cases of prostate cancer in the UK each year and more than 330,000 across European Union countries.
EDX Medical expects its test to deliver exceptionally high accuracy with levels of sensitivity and specificity of between 96-99% across an extended age-range and diverse ethnic groups. By comparison, current standard of care prostate testing, including prostate specific antigen (PSA) tests and biopsies, can be below 50%.
The non-invasive ‘super test’ will detect various sub-types of prostate cancer, as well as determining key features particularly important for patients in non-caucasian higher risk groups.
The test adopts a ‘multi-omics’ approach comprising the assessment of a combination of proteomic, transcriptomic, genetic/hereditary and epigenetic biomarkers combined with additional phenotypic and symptom data by the AI algorithm.
Individually, these biomarkers have all been clinically validated in more than 31,000 prostate cancer samples as well as more than 100,000 non-cancer control samples.
A highly accurate prostate cancer test will provide significant benefits for seemingly well 45-70 year-old men and also for healthcare providers. The increased accuracy should reduce the requirement to run unnecessary MRI scans, and the need for highly invasive digital rectal examinations (DRE) will also be dramatically reduced.
Prof Sir Chris Evans, founder and chief scientific officer of EDX Medical, said: “We have been working intensively in this area and are tremendously excited by what we believe is a truly game-changing test. Every indication thus far shows it will be the most accurate and sensitive screening test available and will be transformative in tackling prostate cancer in men who may have no idea if anything is wrong with them.
“Our integrated approach highlights the potential of combining these molecular signatures, offering a powerful, non-invasive diagnostic tool that can certainly improve clinical outcomes and help personalise treatment for patients. The incorporation of all these biomarkers into routine screening could revolutionise prostate cancer management by enabling earlier detection and more accurate risk prediction. What sets this test apart is the use of so many biomarkers with best-in-class instrument and reagent technology and our bespoke AI algorithm.”
Sir Chris Hoy, who has been diagnosed with prostate cancer and supports campaigns to raise awareness and encourage early diagnosis, said: “Prof Sir Chris Evans and his team encouraged and supported me greatly after my initial diagnosis. I know they have some amazing people and a great commitment to finding better ways to diagnose and treat prostate and other cancers. I now know there is a need for better and more accurate prostate cancer screening tests and I wholeheartedly welcome this initiative by Sir Chris’ EDX.”
Andy Taylor, guitarist with Duran Duran who was diagnosed with prostate cancer at 55, said: “Prof Sir Chris helped me immensely with my cancer treatment and this revolutionary new test from his labs is simply brilliant. To know your prostate cancer status, stage and type and all your genetics when you were unaware there was a problem at all is a life saver and game-changer. It is so accurate and comprehensive, it can spot many early prostate cancers, save many lives and save a fortune in fruitless treatments: this is priceless.”
Dr Mike Hudson, chief executive of EDX Medical said: “I’m confident that the EDX testing strategy will define a new standard for the early detection and characterisation of emergent, prostate cancer, and provide unique insights to guide optimal treatment selection.”
EDX Medical’s scientific team will validate further clinical data over the coming months with a view to launching the test later this year.